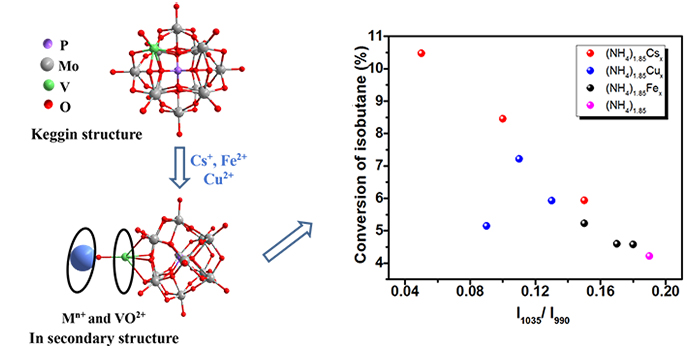
Our group prepared a series of ammonium salts of molybdovanadophosphoric acid with diferent metal substitution , and investigated the catalytic performance of the catalysts in the selectiviey oxdiation of isobutane to methacrylic acid. .The study found that the specific surface area, amount of acid sites, and immigrating amount of V atom in Keggin unit into the secondary structure were strongly dependent on the substituted metal ions and their content. The optimum activity was obtained over (NH4)1.85Cs0.5 catalyst, which could provide a larger surface area , a higher amount of acid sites and more VO2+ species and V2O5 clusters from the immigration of V atom in Keggin structure. In addition, because the replacement catalyst of Cu ions accelerates the electron transfer efficiency and accelerates the catalytic cycle of isobutane oxidation, the desorption speed of MAA is accelerated, and the catalyst exhibits excellent selectivity to MAA.This work has been published in Catalysis Letters.
The article links:https://link.springer.com/article/10.1007/s10562-021-03821-3