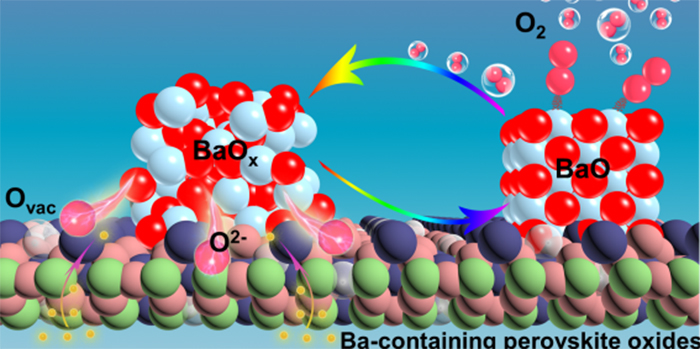
We disclosed a mechanism of oxygen exchange reactions for Ba-containing materials where BaO/BaO2 acts as active sites. Oxygen activation, including oxygen reduction reaction (ORR) and oxygen evolution reaction (OER), is at the heart of many important energy-conversion processes. However, the activation mechanism of Ba-containing perovskite materials is still ambiguous, due to the complex four electron transfer process on the gas-solid interfaces. We directly observe that BaO and BaO2 segregated on Ba-containing material surface participate into the oxygen activation process via the formation and decomposition of BaO2. Tens of times increase in catalytic activities was achieved by introducing barium oxides in the traditional perovskite and inert Au electrodes, indicating barium oxides are critical for oxygen activation. We find BaO and BaO2 are more active than the B-site of perovskite for ORR and OER, respectively, and closely related to the high activity of Ba-containing perovskite. This work has been published in Science Advances, 2022, 8, eabn4072.
The article links:https://www.science.org/doi/10.1126/sciadv.abn4072