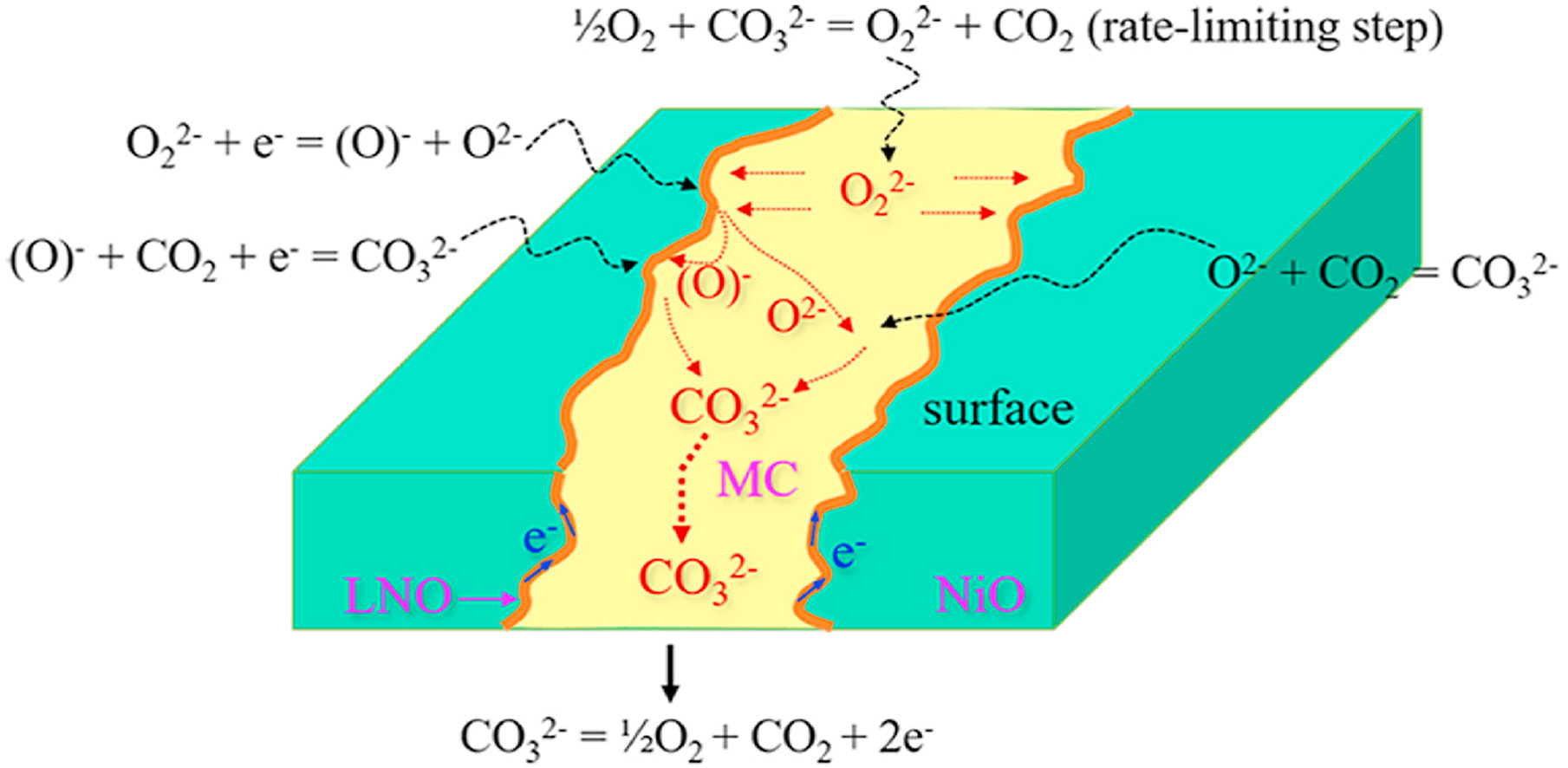
Our group revealed the effect of molten carbonate composition on CO2 permeation mechanism.High temperature dual-phase mixed conductor membranes are promising for CO2 separation and utilization. Most efforts are focused on materials, thickness, microstructural features and surface modifications of the electronic conducting phase. Here, we unveil the effect of the carbonate-ionic conducting phase on CO2 permeation process with a dense NiO-based dual-phase membrane. The results demonstrate that the CO2 flux increases first and then decreases with the increase of Li2CO3 addition amount. The controlling step of CO2 permeation changes from bulk diffusion to surface reaction as much more Li2CO3 was added into the carbonate-ionic conducting phase. Model analysis indicates that the peroxide formation process on the membrane surface is the rate-limiting step, which is closely related to the oxygen solubility of molten carbonate.This work has been published in Journal of Membrane Science.
The article links:https://www.sciencedirect.com/science/article/pii/S0376738821011509