Recently, the research team of Yang Weishen and Zhu Kaiyue in our group has made new progress in the research of cathodes of aqueous zinc-ion batteries, which has brought a major breakthrough to the development of aqueous zinc-ion batteries. This study clarified the core mechanism of capacity decay of vanadium-based cathode materials for the first time, and developed an innovative electrolyte solution, which significantly improved the battery cycle stability. This discovery not only solves the scientific problems that have long plagued the academic community, but also clears the key obstacles for the commercial application of aqueous zinc batteries.
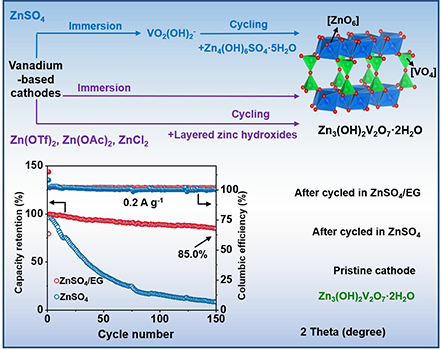
For a long time, aqueous zinc-ion batteries have been regarded as an ideal choice for large-scale energy storage due to their high safety and low cost. Among them, vanadium-based cathode materials have attracted much attention due to their high capacity and excellent rate performance. However, their practical application has always faced the bottleneck problem of poor cycle stability. The traditional view is that vanadium dissolution is the main cause of performance degradation, but this latest study overturned this cognition. The research team confirmed through systematic experiments that the Zn3(OH)2V2O7·2H2O (ZOV) byproduct generated after dissolution is the real "performance decay culprit". This discovery points the way for subsequent technological breakthroughs.
During the research, by preparing high-purity ZOV samples and conducting electrochemical tests, it was confirmed that the Zn2+ storage capacity of the substance was only 1 mAh/g, and it had almost no electrochemical activity. This key discovery explains why vanadium-based batteries experience significant capacity decay in actual use. More excitingly, the team also systematically revealed two different generation pathways for the formation of ZOV, and pointed out that the dissolution of vanadium-based materials is the cause of the formation of ZOV. It laid a theoretical foundation for the subsequent proposal of targeted solutions.
The research team developed an innovative electrolyte design. A new hybrid electrolyte was formed by adding 75% ethylene glycol (EG) to 2M ZnSO4. This design achieves the effect of inhibiting the dissolution of vanadium-based materials and effectively blocks the generation path of ZOV. Experimental data show that the (NH4)0.4V2O5·1.6H2O cathode using this electrolyte has a capacity retention rate of up to 85% after 150 cycles at a current density of 0.2 A/g, which is much better than the 26% of traditional electrolytes.
The above work was recently published in the Chemical Engineering Journal under the title of "Unlocking the performance degradation of vanadium-based cathodes in aqueous zinc-ion batteries". The first author of the work is Li Weijian, a doctoral student in the 504 group of our institute. The work was funded by the National Natural Science Foundation of China, our institute's innovation fund and other projects.
The article links: https://www.sciencedirect.com/science/article/pii/S1385894724052756?via%3Dihub